VERO Biotech Recognized as a 2024 AARC Zenith Award Winner
VERO will receive the prestigious 2024 AARC Zenith Award at the AARC International
Respiratory Congress (AARC Congress 2024) taking place November 20-23 in Orlando, FL
ATLANTA, September 12, 2024 — VERO Biotech Inc, a fast-growing commercial business dedicated to improving the lives of patients through innovative technologies, today announced the honor of receiving the 2024 Zenith Award from the American Association for Respiratory Care (AARC).
Each year, AARC recognizes companies in the respiratory care industry for their support of the profession via the AARC Zenith Award. The AARC Zenith Award is the “people’s choice” award of the respiratory care profession because its recipients are chosen by respiratory therapists across the nation based on the following criteria:
- Quality of equipment, supplies, or services

- Accessibility and helpfulness of sales personnel
- Responsiveness; Service record
- Truth in advertising
- Support of the respiratory care profession
This year, VERO Biotech is honored to be recognized as a 2024 AARC Zenith Award recipient. Out of 300+ companies nominated; VERO Biotech is one of just five to receive the award.
“We are humbled to be recognized by this elite organization and remain committed to the RT community as we provide a high-quality product with the GENOSYL® Delivery System and exemplary service to the respiratory care profession,” said Brent Furse, CEO and President, VERO Biotech.
The American Association for Respiratory Care is the leading national and international professional association for Respiratory Care. They work with RTs to encourage, promote, and facilitate their professional excellence. They do that by advancing the science and practice of Respiratory Care and serving as an advocate for patients and their families, the public, the profession, and each unique and individual respiratory therapist.
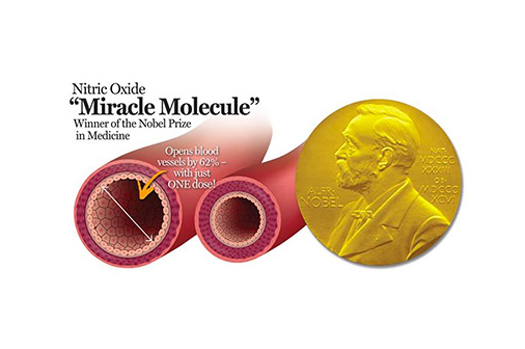
GENOSYL® DS is the first tankless inhaled nitric oxide delivery system approved by the U.S. Food and Drug Administration (FDA). Inhaled Nitric Oxide dilates pulmonary blood vessels and may be used to improve oxygenation in neonates with hypoxic respiratory failure and pulmonary hypertension. Unlike tank-based systems, GENOSYL DS generates and delivers iNO at the bedside using a small disposable cassette. This eliminates the need for hospitals to manage large, cumbersome tanks, helps to simplify clinical workflow, and streamlines patient care.
About GENOSYL®
Indication
GENOSYL (nitric oxide) gas, for inhalation, is indicated to improve oxygenation and reduce the need for extracorporeal membrane oxygenation in term and near-term (>34 weeks gestation) neonates with hypoxic respiratory failure associated with clinical or echocardiographic evidence of pulmonary hypertension in conjunction with ventilatory support and other appropriate agents.
Important Safety Information
- GENOSYL is contraindicated in the treatment of neonates dependent on right-to-left shunting of blood.
- Abrupt discontinuation of GENOSYL (nitric oxide) gas, for inhalation may lead to worsening oxygenation and increasing pulmonary artery pressure.
- Methemoglobin levels in the blood increase with the dose of nitric oxide; following discontinuation or reduction of nitric oxide, methemoglobin levels return to baseline over a period of hours.
- Methemoglobin, NO2, and PaO2 should be monitored during nitric oxide administration.
- In patients with pre-existing left ventricular dysfunction, GENOSYL may increase pulmonary capillary wedge pressure leading to pulmonary edema.
- The most common adverse reaction is hypotension.
- Nitric oxide donor compounds may have an additive effect with GENOSYL on the risk of developing methemoglobinemia.
- GENOSYL must be administered using a calibrated GENOSYL Delivery System. Only validated ventilator systems or nasal cannulas should be used in conjunction with GENOSYL.
Please visit www.vero-biotech.com for the full Prescribing Information for GENOSYL.
About GENOSYL® DS
GENOSYL DS is a tankless system engineered with redundant backup features that delivers a constant concentration of inhaled nitric oxide gas to patients with an easy-to-use interface and portability features. This proprietary delivery system eliminates the need for large nitric oxide tanks and the associated logistical burden. GENOSYL DS recently received FDA approval for its innovative dual-cassette design and secondary adaptive sensor technology to further optimize patient care.
About VERO Biotech
VERO Biotech Inc. is a privately held company headquartered in Atlanta, Georgia focused on saving lives, alleviating suffering, and improving health economics in the neonatal intensive care and the acute care hospital communities.
Forward Looking Statements
This press release and any statements of representatives of VERO Biotech Inc. related thereto that are not historical in nature contain, or may contain, among other things, certain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements may include, without limitation, statements with respect to VERO Biotech’s plans, objectives, projections, expectations and intentions and other statements identified by words such as “projects,” “may,” “will,” “could,” “would,” “should,” “believes,” “expects,” “anticipates,” “estimates,” “seeks,” “intends,” “plans,” “potential” or similar expressions, including statements with respect to the potential benefits, advantages or market opportunity of the products. These statements are based upon the current beliefs and expectations of VERO Biotech’s management and are subject to significant risks and uncertainties. Actual results may differ significantly from those set forth in the forward-looking statements. These forward-looking statements involve certain risks and uncertainties that are subject to change based on various risk factors (many of which are beyond the control of VERO Biotech).
For information, please visit www.vero-biotech.com or contact us at contactus@vero-biotech.com.
SOURCE VERO Biotech Inc.