Innovation in Inhaled Nitric Oxide Delivery
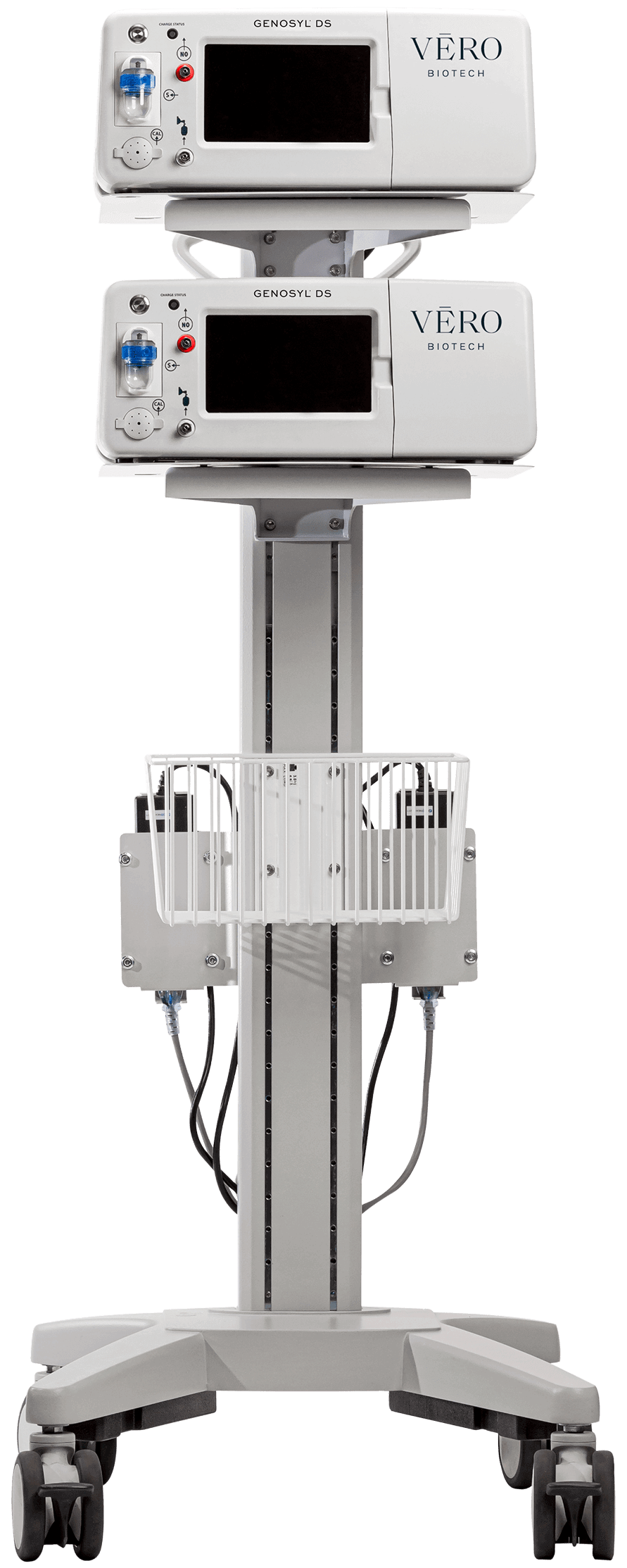
This product demonstration highlights the basic functions and operation of the GENOSYL® Delivery System.
This product demonstration highlights the basic functions and operation of the GENOSYL® Delivery System.
Persistent pulmonary hypertension of the newborn (PPHN) with hypoxic respiratory failure is a serious condition in term and near-term (>34 weeks) newborns. PPHN occurs as a primary developmental defect, when there is not a successful transition of gas exchange to the lungs upon birth, and high pulmonary vascular resistance (PVR) results. PPHN can also occur as a condition secondary to other diseases such as meconium aspiration syndrome (MAS), pneumonia, sepsis, hyaline membrane disease, congenital diaphragmatic hernia (CDH), and pulmonary hypoplasia. In these states, PVR is high, which results in hypoxemia secondary to right-to left shunting of blood through the patent ductus arteriosus and foramen ovale.
Inhaled nitric oxide has revolutionized the treatment of PPHN by dilating the pulmonary vasculature, providing improved oxygenation and a reduced need for invasive extracorporeal membrane oxygenation (ECMO). This condition is currently the only FDA-approved indication for inhaled nitric oxide, thus being the standard of care in the treatment of PPHN.
Persistent pulmonary hypertension of the newborn (PPHN) with hypoxic respiratory failure is a serious condition in term and near-term (>34 weeks) newborns. PPHN occurs as a primary developmental defect, when there is not a successful transition of gas exchange to the lungs upon birth, and high pulmonary vascular resistance (PVR) results. PPHN can also occur as a condition secondary to other diseases such as meconium aspiration syndrome (MAS), pneumonia, sepsis, hyaline membrane disease, congenital diaphragmatic hernia (CDH), and pulmonary hypoplasia. In these states, PVR is high, which results in hypoxemia secondary to right-to left shunting of blood through the patent ductus arteriosus and foramen ovale.
Inhaled nitric oxide has revolutionized the treatment of PPHN by dilating the pulmonary vasculature, providing improved oxygenation and a reduced need for invasive extracorporeal membrane oxygenation (ECMO). This condition is currently the only FDA-approved indication for inhaled nitric oxide, thus being the standard of care in the treatment of PPHN.
GENOSYL® Delivery System approved for transport in ground, fixed-wing & rotary-wing.
GENOSYL® Delivery System is conditionally compatible up to the 100 Gauss Line with 1.5 Tesla and 3.0 Tesla Diagnostic Imaging Environments.
GENOSYL® Delivery System is conditionally compatible up to the 100 Gauss Line with 1.5 Tesla and 3.0 Tesla Diagnostic Imaging Environments.
VERO Biotech actively validates new equipment on an ongoing basis with the GENOSYL® DS. The following list outlines various ventilators and ancillary equipment that have been validated with the GENOSYL® Delivery System.