A Simplified, Tankless Solution for Inhaled Nitric Oxide (iNO). Designed to help improve the efficiency of patient care.

Experience GENOSYL® DS at AARC Congress 2025
Clinician-Driven Innovation for Respiratory Care See it in Action at Booth #1247
One device for all inhaled nitric oxide (iNO) needs.
GENOSYL DS provides seamless integration wherever iNO is needed. The only iNO device with approval for use in all areas of care, without requiring device changes.

Seamless Integration Wherever iNO is Needed.
GENOSYL® DS enhances workflow and streamlines operations at every nitric oxide touchpoint.
Quick Simplified Setup
Simplified hassle-free setup and dose initiation in less than 2 minutes.
Precise Dose Delivery
Smart Feedback System™ ensures the set nitric oxide dose is accurately maintained, based on a sampled value, regardless of changes in flow.
Compatibility
Flexible use with multiple respiratory devices across areas of care.
Single-Use, Disposable Cassette and Accessories
Storable at bedside, negates tank management, and eliminates reprocessing.
Seamless Manual Bagging
Transition with the push of two buttons and no complicated calculations.
GENOSYL® DS is the only device approved for both intra- and inter-hospital transport across ground, fixed-wing, and rotary-wing environments.
Vigorously Tested
Tested across all appropriate standards per FDA and FAA requirements, including static pull and EMI / EMC testing.
Custom Transport Mount
Featuring a quick-connect plate designed to interface with common sled connections.
Compact Design
Ensures easy access during transport.
A Single System
Allows for simple patient handoff from the acute care setting to the transport vehicle.
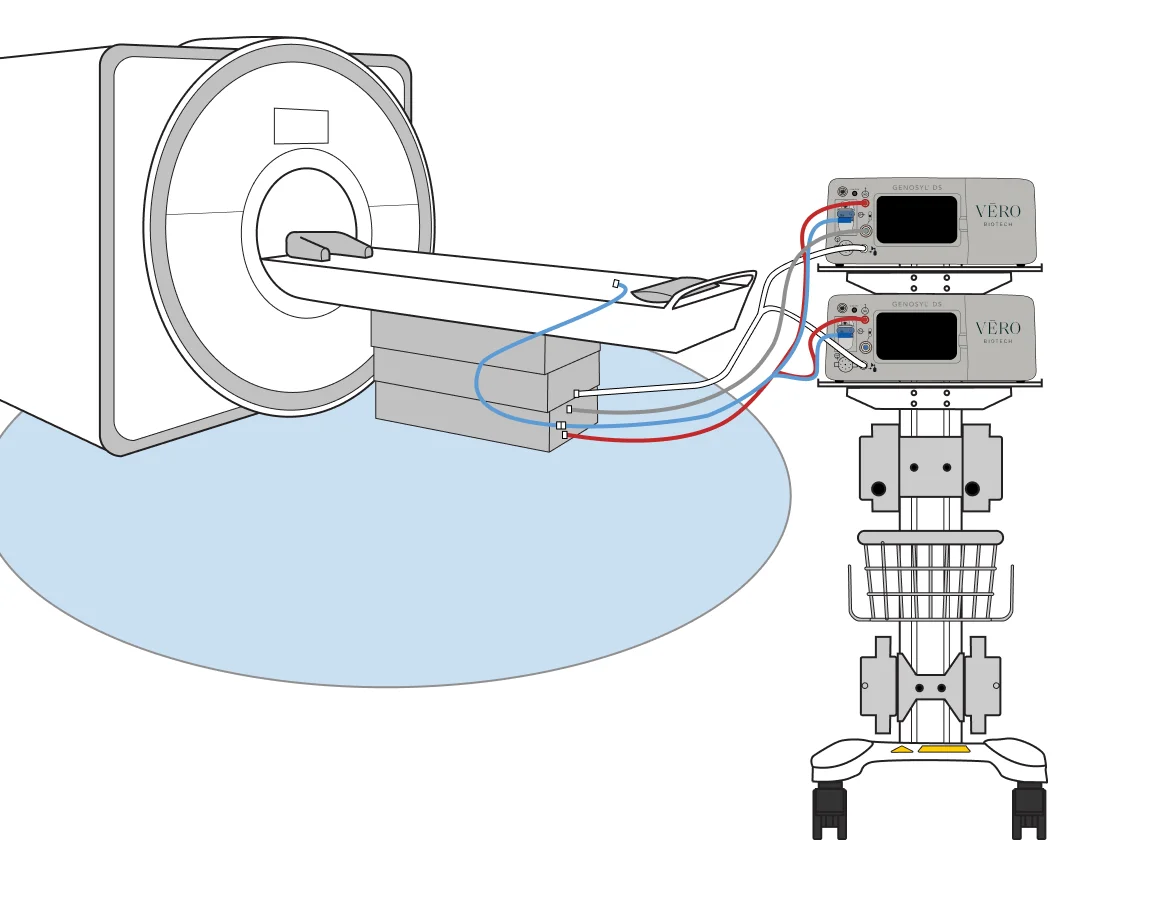
GENOSYL® DS is conditionally compatible up to the 100-Gauss Line.
Compatibility
Compatible with 1.5 Tesla and 3.0 Tesla diagnostic imaging environments.
Fixed Gauss Alerts
Fixed gauss alerts will alarm, providing safety feedback if the system is too close to the scanner (greater than 100 gauss).
Maintains System Position
Sample line extension maintains system position outside the exclusion zone, allowing uninterrupted nitric oxide dosing when MR scanner is in use.
GENOSYL® DS is the only device approved to accurately deliver inhaled nitric oxide in Low-Flow Anesthesia environment
Streamlines Workflow
Streamlines workflow with simple setup and dose initiation, proven to be able to be completed by Anesthesia providers.
Supports Physician’s Preference
Eliminates the need to change practice to deliver inhaled nitric oxide.
Achieves Significant Cost Savings
When used in low-flow anesthesia, the amount of anesthetic agent used in recirculating systems is reduced, thereby reducing cost.1 2
GENOSYL® Delivery System
Engineered for Operational Efficiency, Accuracy, and Safety
The power of inhaled nitric oxide in a pocket-sized GENOSYL® DS Cassette.
Compact, 16 oz Cassette available at point of care for easy clinician access.
Patented technology generates inhaled nitric oxide on demand while eliminating the burden of tanks.
Simplified Cassette activation allows for immediate initiation of the dose.
Automatic Cassette transition ensures continuous, uninterrupted dosing, and minimal device management time.
No purging required, reducing exposure to NO2.
Proprietary Smart Feedback System™ ensures precise, accurate dosing of inhaled nitric oxide to every patient, every time.
Controls dose independent of ventilator settings and flow changes.
Samples iNO at the patient and adapts to achieve precise dose control.
Enables accurate delivery of low doses in low-flow environments.
Precision in low dosing assists in efficient weaning of iNO, with the goal of improving patient outcomes, shortening ICU stays, and reducing cost.
Transforming the Delivery of Inhaled Nitric Oxide
Redundant back-up Console ensures continuous iNO delivery.
Automatic Cassette transition reduces potential for clinical errors, such as abrupt discontinuation.
Seamless manual bagging transition (no calculations required).
Continuous dose monitoring to ensure precise delivery and safe continuity of care wherever iNO is needed.
Partnership365™ Service and Support: We’ll Be There, Because We’ve Been There
24/7/365
Supporting innovation and change, our experienced Registered Respiratory Therapists provide 24/7/365 support and customized partnerships to ensure success.
Team
Customers will have access to the largest clinical support team of registered respiratory therapists with a minimum of 10 years of bedside experience.
Medical Information
Experts provide concise, accurate, and clinically relevant data to your medical requests.
Inventory Management
Support team provides personalized monitoring and management of equipment and inventory.
Technical Support
Specialized assistance from GENOSYL Delivery System technical experts.
Training and Education
Comprehensive system onboarding, live training by respiratory therapy clinical experts, and exclusive on-demand access to educational videos and electronic resources via the online VERO Knowledge Center.

Schedule a Demo
Experience GENOSYL® DS at AARC Congress 2025
Clinician-Driven Innovation for Respiratory Care See it in Action at Booth #1247